Cold stamping medicine foil 8079 8021
The aluminum foil substrate for cold-formed medicine foil is a specially treated aluminum foil material with high density and barrier properties. It can effectively block external factors such as moisture, air, and light, thereby protecting medicines from contamination and deterioration. In addition, the aluminum foil substrate also has good formability and heat sealing properties, which can meet the needs of different medicine packaging.
The aluminum foil substrate for cold-formed medicine foil usually adopts alloy grades such as 8021 and 8079, and is usually supplied in O state (soft state) to facilitate subsequent stamping and heat sealing operations. It has the advantages of high cupping value, high heat sealing strength, no pinholes, and good sealing. It is a supplier of raw materials for cold-formed medicine aluminum foil for various foreign pharmaceutical packaging companies
Structure of cold-formed aluminum foil:
Cold-stamped aluminum foil is usually a multi-layer composite structure, which is composed of aluminum foil and other barrier materials to ensure that the packaging has excellent mechanical strength and barrier properties. The common structure is three layers:
OPA (nylon) layer + aluminum foil layer + PVC (or PP) layer
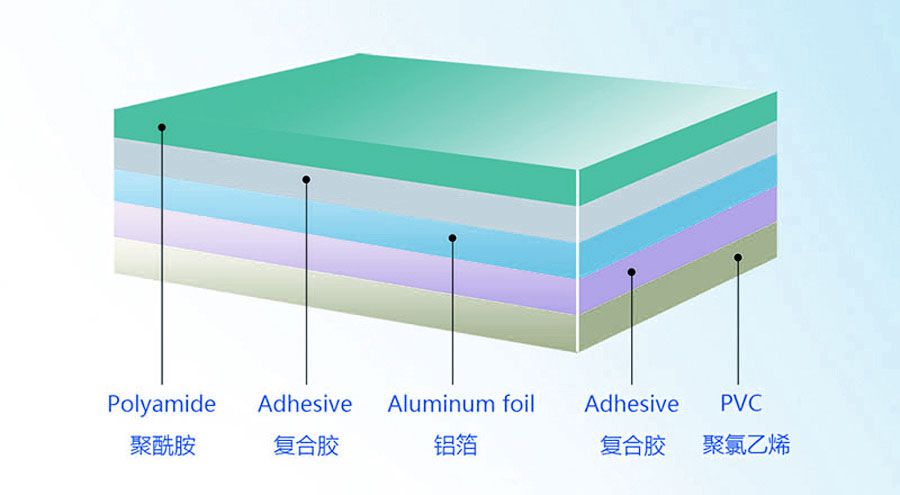
Nylon (OPA) layer: outer layer material, providing high tensile strength and impact resistance for cold stamping aluminum, making it not easy to break during cold forming, and also providing certain barrier properties.
Aluminum foil layer (Al): middle layer, usually pure aluminum foil with a thickness of 45-60 microns, is the main barrier layer, with extremely high moisture resistance, oxygen resistance and light resistance, effectively protecting the stability of drugs.
Polyvinyl chloride (PVC) or polypropylene (PP) layer: inner layer material, directly in contact with drugs, with good heat sealing and chemical resistance to ensure the safety of drugs and the sealing of packaging.
Common composite structure examples:
OPA 25 μm / Al 45 μm / PVC 60 μm
OPA 15 μm / Al 20 μm / PVC 50 μm
OPA 25 μm / Al 30 μm / PVC 60 μm
8021 8079 cold-formed aluminum foil performance advantages:
1. Excellent barrier properties: effectively block gases (such as oxygen, water vapor) and light radiation, protecting drugs from external influences.
2. High sealing: compounded with a variety of materials to form a firm sealing structure to ensure the stability and safety of drug packaging.
3. Excellent mechanical properties: high strength and high toughness, heat and low temperature resistance, able to withstand various stresses and deformations.
4. Easy to process and form: suitable for various processing techniques, meet different drug packaging needs, and adapt to automated packaging equipment.
5. High safety: non-toxic, odorless, environmentally friendly, will not pollute drugs, and has good chemical stability.
6. Protect drug quality: keep drugs dry and moisture-proof, extend shelf life, and good light-shielding properties to prevent deterioration.
7. Easy to carry: light and easy to carry, convenient for patients to use.
Specifications of cold-formed aluminum foil for pharmaceutical packaging materials:
| Alloy | 8021, 8079 |
| Temper | O |
| Thickness | 0.025-0.08mm |
| Width | 100-1600mm |
| Length | C |
| ID | 76mm |
| Application | Used for heat sealing with hard aluminum foil to package capsules, pills or tablets. |
| Features | 100% barrier to water vapor, gas, light, etc. |
| Packaging | Standard seaborne export packaging-fumigation wooden box |
| MOQ | 1-3 tons |
| Chemical composition(%): | |||||||||||
| Alloy | Al | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Single | Total |
| 8079 | Remainder | 0.20 | 0.30 | 1.0-1.5 | 0.10 | 0.6-1.3 | 0.10 | 0.25 | 0.10 | 0.05 | 0.15 |
| 8021 | Remainder | 0.15 | 1.2-1.7 | 0.05 | - | 0.05 | 0.05 | 0.10 | 0.08 | 0.05 | 0.15 |

Application scenarios
Cold-formed pharmaceutical foil is widely used in the following occasions:
Blister packaging of solid drugs such as tablets and capsules
High barrier packaging of injections and granules
Pharmaceutical packaging that requires long-term storage or special storage conditions
Surface processing requirements for cold-formed pharmaceutical aluminum foil
1. Plate shape: The plate shape of the aluminum foil substrate should be flat, without obvious waves, warping, etc., to ensure the accuracy and stability of stamping.
2. Surface quality: The surface of the aluminum foil substrate should be clean, free of oil stains, scratches, bright spots and other defects to ensure the neatness and beauty of the pharmaceutical packaging.
3. Holes and pinholes: The aluminum foil substrate should be free of holes and pinholes to avoid contamination of the drug during the packaging process.







Contact Us